Hope for HIE families have been a part of every single landmark clinical trial relating to neonatal and pediatric-acquired Hypoxic Ischemic Encephalopathy, globally. It is because of the tenacity, willingness to participate and HOPE in the future that we know as much as we do about HIE.
But, there is so much more we need to do.
Now, more than ever before, there is tangible HOPE on the horizon for additional therapeutics and changes in clinical care to decrease the incidence and impact of HIE. Whether it’s a new discovery, or looking at existing therapies that may work with HIE, there’s a lot to consider from the patient-family side when going through the process to decide to participate in clinical trials.
Hope for HIE regularly partners with researchers – academic, industry, nonprofit & for profit entities – from all over the world as a part of our advocacy to decrease the incidence and impact of neonatal and pediatric-acquired HIE to improve the quality of life for children and families. We have a rigorous discovery process to ensure collaborations and partnerships are aligned with our organizational values and mission.
Explore more about Clinical Trials:
Choosing to participate in a clinical trial or research study can be a complex decision, especially with neonatal HIE, and the urgency that can come with a decision in the midst of a complex, high stress medical situation.
We want to ensure HIE families, whether brand new to this journey, or those seeking clinical trials for investigational medications and therapies, or research studies, have unbiased information and feel confident in making the decision to enroll, or not enroll, in a clinical trial. We’ve created some educational resources below, as well as curated links to HIE-related clinical trials.
Research is not just the responsibility of scientists and doctors. It is important that patients and families participate to whatever extent they are able. Research can’t move forward without participation of patients and families!
Patient-families can participate in research in several ways, including:
We’ve brought together the best resources we know of, reviewed by our Medical Advisory Board, to help the HIE community understand as much as possible about participating in research and potential treatments, medications, therapies and interventions aiming to improve the quality of life for children and families impacted by neonatal and pediatric-acquired Hypoxic Ischemic Encephalopathy.
Clinical trials are research studies performed in humans with a medical condition like HIE. They scientifically evaluate a medical or surgical intervention. They are the primary way that researchers find out if a new treatment, like a new drug, diet, or medical device is safe and effective.
Without volunteers, clinical trials cannot exist and we would not be able to find new treatments for HIE and its wide variety of impacts. Families who participate in clinical trials are on the cutting edge of helping us to find better treatments and cures. The reason we have treatments for HIE today is due to the fact that HIE families participated in past trials. We are forever grateful for their contribution.
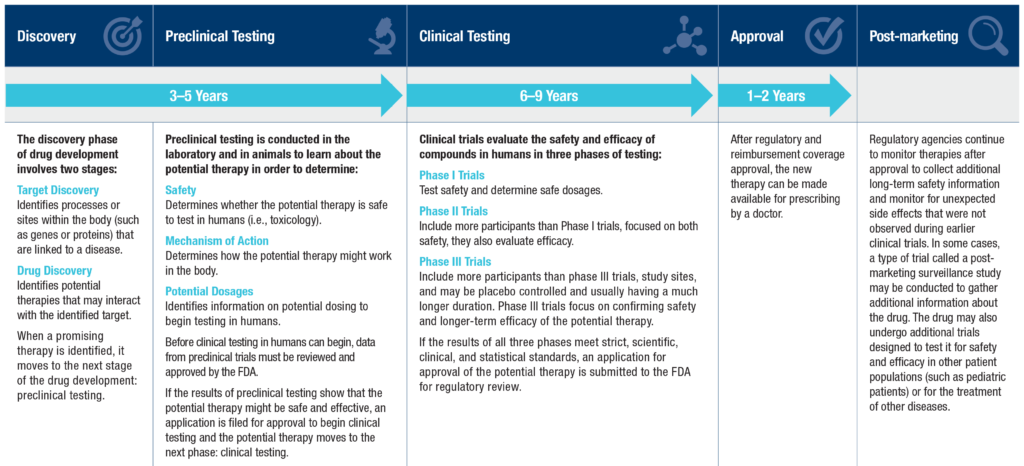
Any new medication, therapy, or device has a development lifecycle, a thorough process to look at how effective and safe a potential therapy might be.
Discovery
This is when something is discovered by scientists, usually in a lab, or a currently approved medication is looked at for other uses.
Preclinical Testing
Because the brain is so complex, you might be asking ‘How does preclinical testing work to ensure new therapies are safe to try in humans?’ Some people might not like this part, but it’s necessary for scientific discovery and ensuring safety for fragile human babies — it’s animal testing. For HIE, there are several different animal models that are used to replicate HIE. Most commonly, the Vannucci model is used in mice and rats; this model was the basis for the therapeutic hypothermia trials. Other informative animal models include sheep and pigs. There are also labs studying human cells in a dish or organoids that can teach us about specific genes and human brain cell types that are impacted by hypoxia. We need to learn from all of these models together to discover the therapies that will be safest and most effective to help babies with HIE have better outcomes.
This phase includes determining safety, looking at mechanisms of action (how it works in the body), and potential dosages to see what might be the most effective.
Once results can be repeated with consistency, it’s time to show “proof of concept” and take it to the regulatory phase, working with the host country’s regulatory body – the FDA, European Medicines Agency, etc.
Preclinical testing can take 3-5 years or longer!
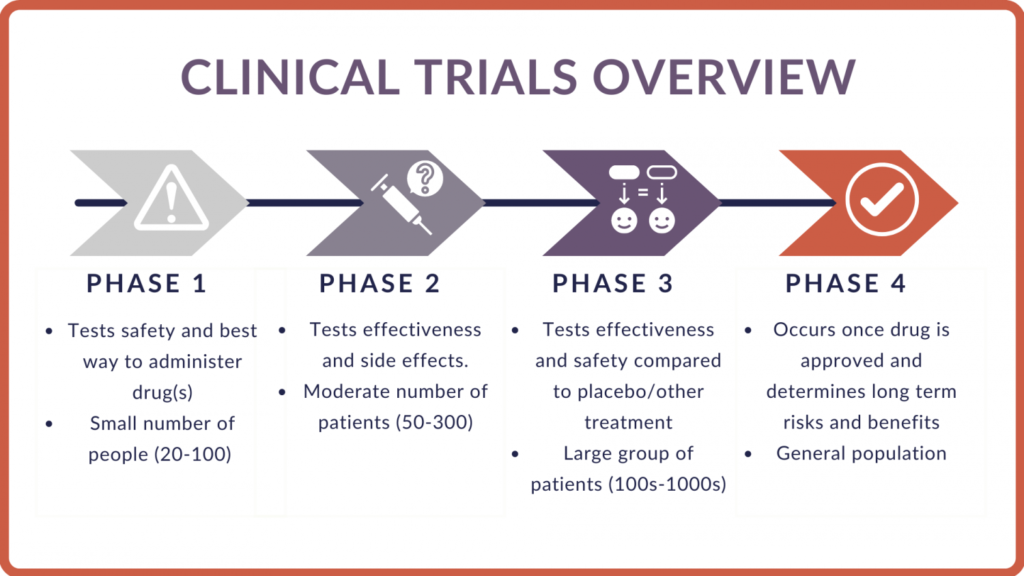
Phase 1 Studies:
In phase 1 of a clinical trial, researchers test a new drug or treatment in a small group of healthy volunteers and/or people with the disease for the first time and they evaluate its safety, determine a safe dosage range, and identify side effects.
This is done after the medication or therapy has gone through rigorous pre-clinical lab testing, worked through regulatory processes for safety, and adjustments made to get approval to begin phase 2 studies.
Phase 2 Studies:
In phase 2, a drug or treatment is given to a larger group of people to see if it is effective for the intended purpose and group of people, and to further evaluate its safety. Participants are followed for set time period (usually 2-3 years).
Phase 3 Studies:
In phase 3, the drug or treatment is given to large groups of people to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely. More than one Phase III study may be required before a New Drug Application (NDA) may be submitted to the FDA. Participants are followed for set time period (usually 2-3 years).
Regulatory Approval:
Once the FDA, or other regulatory body, accepts a filing for the approval of a new treatment, the regulators must complete its review process within 10 months in most cases. The date at the end of the review period is referred to as the Prescription Drug User Fee Act (PDUFA) date.
Phase 4 Studies:
In phase 4, studies are done after the drug or treatment has been FDA-approved and distribution to clinical use is done, working with clinicians and families. The goal is to gather information on the drug’s effect in various populations and identify any side effects associated with long-term use.
The design of a clinical trial describes a sequence and structure of activities aiming to reveal a cause and effect relationship – defined in the research question. All trials begin with a single group of participants (the COHORT) who are selected to represent a defined disease population.
Common clinical trial designs include single-arm trials, placebo-controlled trials, crossover trials, factorial trials, noninferiority trials, and designs for validating a diagnostic device. The choice of the structural design depends on the specific research questions of interest, characteristics of the disease and therapy, the endpoints, the availability of a control group, and on the availability of funding.
Randomized Control Trials
The randomized control trial (RCT) is a trial in which subjects are randomly assigned to one of two groups: one (the experimental group) receiving the intervention that is being tested, and the other (the comparison group or control) receiving an alternative (conventional) treatment.
Comparative Effectiveness Trials
Comparative effectiveness research is the direct comparison of existing health care interventions to determine which work best for which patients and which pose the greatest benefits and harms.
What’s the difference?
Randomized Control Trials (RCT) determine efficacy or how well a treatment works under ideal conditions. Comparative Effectiveness Research (CER) studies examine effectiveness by comparing one or more treatments, procedures or medications to determine what works best for which patients under real world conditions.
Probability of Receiving a Medication/Intervention in Testing
Each trial or study will have a different probability of the patient receiving the intervention. IT’s important to have a “placebo” group to test that something is safe and effective. The study wouldn’t be allowed if it was known the therapy was effective and there may be risks associated with the treatment. By having randomization, we can fairly see who lands in each “arm” of the trial — the placebo/control group, or the group receiving the intervention/medication.
For Neonatal HIE, or HIE that happens around birth, timing is very important to be able to test the effectiveness of potential new medications and therapies. These happen during the critical care in a Neonatal Intensive Care Unit (NICU).
Neonatal HIE babies typically require many interventions to stabilize their bodies from the effects of the causes of neonatal HIE. Because HIE can impact various body systems, there may be different clinical trials for specific impacted systems or issues that are common with HIE such as respiratory support, seizures, kidney function, and other common short-term impacts.
Currently, therapeutic hypothermia (brain cooling) is the only approved intervention for neonatal HIE and the whole body system, and has strict criteria for which babies may benefit. This therapy has to be started within six hours of birth, and currently is only approved through worldwide regulatory bodies for moderate to severe presenting babies on the SARNAT scale, with several studies looking into the effectiveness of its use in mild HIE.
HIE is the leading cause of neonatal seizures, and many babies require anti-seizure medications to control these seizures, allowing the brain to calm down and rest and recovery to take place.
Timing in Neonatal Clinical Trials for HIE
New therapeutics and medications are working through the research process, and the most common HIE treatment options that are moving to clinical trials are all being tested first with cooling. That means the timing to participate in these trials is very short, as cooling has to be initiated within six hours of birth.
That may seem very short. If you are one of the families considering enrolling your baby in this short window for a clinical trial, know that we understand the complexity of how you may be feeling. Also know that there have been thousands of families that were a part of the discovery and reason why we know cooling is effective.
We are here for you – now and into the future, with resources to support you. Once the dust settles, we can connect you to other families who have participated in clinical trials for additional support.
Pediatric-Acquired HIE & Clinical Trials in the PICU
Pediatric HIE may also have timely clinical trials that occur at the onset of a pediatric-acquired HIE diagnosis, requiring quick decisions for enrollment at the time of critical care in a Pediatric Intensive Care Unit (PICU).
Clinical Trials in Outpatient Care
Outside of intensive/critical care, there is a significant history of clinical trials for the many impacts of HIE such as epilepsy, cerebral palsy, vision, cognition, autism, kidney and other body systems.
There is also work being done to look at therapeutics that may be safe and effective given outside of a critical care timeframe.
Each clinical trial has its own design and endpoints. For most, the process works like this:
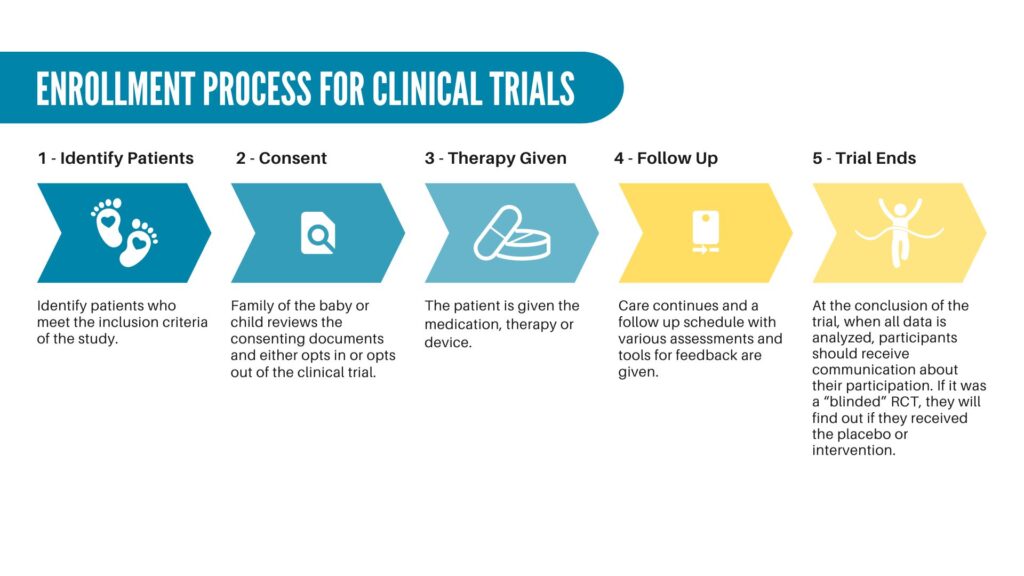
Identify patients who meet inclusion criteria
Criteria is set for the anticipated cohort, and sites that are a part of the clinical trial will work to identify those patients who meet the criteria, and approach them about participation.
Informed Consent
What may seem like a book of information is typically presented to consent in a patient. For neonatal and pediatric trials, this is presented to the family members to review and sign on to have their baby or child participate. It outlines a lot of legal and regulatory statutes to learn about the trial process, the potential risks, and the follow up care and evaluations as a part of the enrollment.
Therapeutic/Medication Dosage
After signing the consent, the therapy, medication or device will be administered. Sometimes in medication trials, they will randomize dosages to see what may be the most effective.
Follow Up
You will work with the enrolling site/hospital to find out about what is expected with follow up. This is equally important to your enrolling in the study! Follow up allows researchers to track the safety, effectiveness and outcomes.
These are some of the medical and technical words are used by researchers and other professionals involved with clinical trials.
Assent—When a child agrees to be in a study.
Blinding or masking—When participants and usually study investigators do not know which medicine or treatment participants are getting until the end of the trial period.
Clinical Equipoise – Clinical equipoise means that when scientists compare two or more groups in a study, they need to make sure each group has an equal chance of seeing an impact from the treatment they’re testing. They decide this based on real scientific evidence that proves the treatment works, not just personal opinions or guesses.
Clinical Trial – A clinical trial involves the study of the effect of an investigational drug/any other intervention in a defined population/participant.
Control Group – A group of people who are used for comparing with another group (intervention group) in an experiment/clinical trial. They receive standard treatment or a placebo (see below).
Informed consent—Parents’ permission for their child to join a research study after they have read an informed consent form, spoken with the investigators, read other materials about the study, and asked questions.
Interventional study—Another name for a clinical trial.
Investigators—People doing the research study. People on the research team may include doctors, nurses, research coordinators, social workers, and other health care professionals. Each clinical study is led by a principal investigator, who is often a medical doctor.
Institutional Review Board (IRB)—An independent committee who ensures that clinical trials are ethical and that the rights of the people in the study are protected. Each clinical trial in the United States must be approved and monitored by an IRB. Members of an IRB include doctors, statisticians, and members of the community.
“Off-Label” Drug Usage – Use of a medicine in a way that has not (yet) been approved by regulatory authorities for a specific age group, dosage or how it is given e.g. as an injection or orally.
Observational Study – A study where investigators observe the effect of a risk factor, diagnostic test or treatment without intervening. Groups are created based on the existence of a health problem e.g. high blood pressure or obesity.
Participants, subjects, or cohort —Volunteers who enroll in a research study.
Placebo—A pill, liquid, or powder without active medicine in it. Placebos are given to children only when withholding treatment poses minimal risks.
Protocol—Detailed plan of the research study.
Randomization—A way to choose what treatment each person in a study gets so that there is less chance of bias. It’s like flipping a coin or rolling dice—the results are random and are by chance, not choice.
Research Study — A Study is not necessarily giving a therapeutic, medicine or intervention. They may want to track certain things based on specific questions or focus areas. Participating is very important to gain a better understanding of the focus area.
Terms were aggregated from American Academy of Pediatrics & EFCNI resources
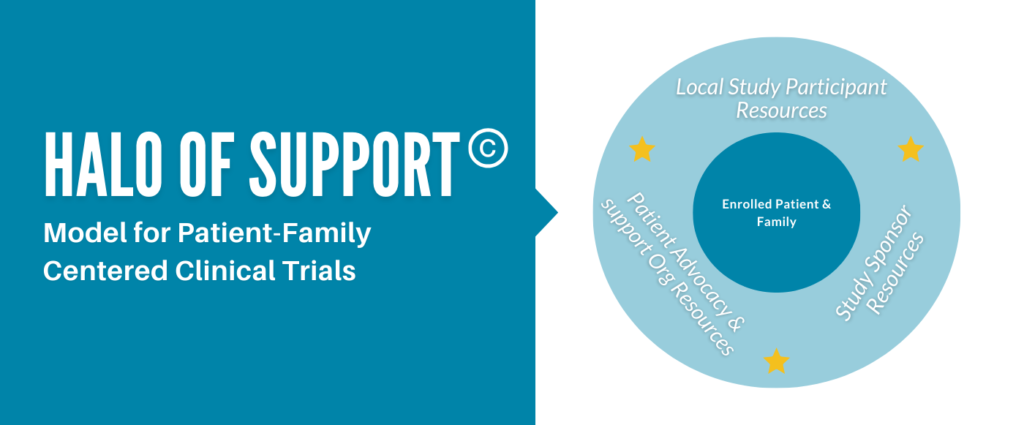
Family participation is KEY in clinical research.
Hope for HIE has developed a comprehensive support framework working with researchers — from early stage preclinical research through regulatory approvals and into clinical trials. We ensure patient-family perspectives are drivers in setting research agendas, elevating what endpoints matter most to families.
We also know the psychosocial support that is needed in the HIE journey, and how participating in clinical trials can be helpful and also confusing and add to the complexity.
Hope for HIE’s proprietary Halo of Support model combines the insights and best practices in communication, patient-family engagement and support with cutting edge science and research to ensure families participating in trials have the long term support needed, and researchers are able to get the data needed to accelerate breakthroughs.
Hope for HIE is here for families, whether a study is engaged in our model of support or not, to connect you into our community, programs and services.
How old does my child have to be to participate?
Each trial or study will have specific inclusion criteria for the age limits to participate.
Do I have to travel somewhere to participate?
Depending on the trial or study, it may be in a specific location where you may have to travel to have your child participate.
I’m feeling overwhelmed after enrolling in a trial or study. Who can help guide me?
Please reach out to our social worker or child life specialist who will be able to talk through concerns. Sometimes families want to connect to others who have enrolled their children in a trial or study, and we have many families you can connect with! Head over to HIE.Support to reach our team.
Will my child have to go through painful procedures?
Each trial or study has specific activities that follow a timeline of care and follow up. Usually researchers are working to minimize pain and discomfort, but each child’s needs are different and if you have concerns about a procedure, imaging, or therapy, it’s important to let the team know. You can always reach out to Hope for HIE’s Child Life Specialist to talk through concerns about pain, sensory management, etc. by heading to HIE.Support.
My child has other diagnoses than just HIE, can we still participate?
Each trial or study has specific inclusion or exclusion criteria which will guide if your child is eligible based on their medical history and diagnoses.
How much time will participating in a trial or study take?
Each trial or study has different time commitments. Some will have follow up appointments in person or virtually.
There are many resources for learning more about active clinical trials for HIE and the many subsequent diagnoses associated with it. We’re working to build out our clinical trials and research online hub here so families can more easily access, learn about, and participate in the many clinical trials aimed at improving outcomes for our children and families.
Hope for HIE is engaged in the following actively enrolling trials as a comprehensive support partner. This does not mean we make any claim to the safety or efficacy of the treatment, medication or intervention, but we are here to provide longitudinal support for families to understand more about clinical trials, the specific trials they may enroll in that we are collaborating on, and access support, with or without enrollment :

A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Preliminary Efficacy of RLS-0071 in Newborns With Moderate or Severe Hypoxic-Ischemic Encephalopathy Undergoing Therapeutic Hypothermia (STAR)
(If you are currently enrolled in the STAR study, be sure to sign up for study-specific support here.)

Cool Prime Comparative Effectiveness Study for Mild HIE (COOLPRIME)
There are many resources to learn more about active clinical trials. There are many resources to pull from to learn about available studies. We recommend Clinicaltrials.gov as a starting point. It aggregates global studies.
There are a few different search terms that apply for HIE-specific studies:
European Union Database – Neonatal Encephalopathy & Neonatal HIE Clinical Trials:

The SURV1VE trial aims to compare methods of neonatal resuscitation (CPR) to potentially improve outcomes and see if the preclinical data and research matches human babies. Because of the critical timeframe, and difficulty in predicting which baby may need CPR at birth, the study coordinators aim to get parental perspectives to help with understanding the best way to consent and communicate about the study to families.
You can help by filling out the survey from the research team!

We are inviting you to participate in a brief research survey (JHM IRB00408428) to better understand regarding healthcare and school access, utilization, barriers, and satisfaction of children with long COVID.
You are being sent this survey because you belong to one of three groups: a) families of children with long COVID; b) families of children with intellectual, physical, or developmental disabilities; and c) educators and school personnel. This survey is entirely voluntary and any care at the Kennedy Krieger Institute and Johns Hopkins will not be affected if you decide not to participate. You can be compensated with a $15 gift card for the completion of this survey. The survey should take 15 minutes or less to complete. We thank you in advance for your participation. If you have any questions or concerns, you can contact Laura Malone MD PhD (Principal Investigator) (malonela@kennedykrieger.org).

SPRINT: Sparking Potential, Revealing Infant Neurocognitive Traits Research Study
Dr. Melisa Carrasco McCaul in the University of Wisconsin-Madison TREES lab is launching research focused on the long-term cognitive development of children, including healthy infants and those who had a perinatal brain injury such as HIE.
Study Format:
As part of this research study, your child will complete an online game and assessments. Parents will be asked to fill out questionnaires remotely.
Enrollment Criteria:
Time Commitment:
Four virtual visits of 45-60 minutes per visit. Patient-families are compensated for their time.
Epilepsy impacts roughly 50-60% of children with HIE across the age and outcome spectrum. HIE is the leading cause of neonatal seizures, the leading non-genetic cause of Infantile Spasms and the rare epilepsies, as well as later childhood onset epilepsy at the sleep/wake cycle.
We hope these resources to look into clinical trials for epilepsy lead to greater treatment options, and success in decreasing the impact for total seizure control and elimination.
Clinical Trials & Research Studies for Epilepsy
You can find a comprehensive list of epilepsy clinical trials at CenterWatch I Connect Clinical Trials Listing for Epilepsy. This list is a subset of available epilepsy clinical trials that can be found on ClinicalTrials.gov
The Lennox-Gastaut Syndrome Foundation is at the forefront of patient-family partnerships for research with LGS. You can learn more about available trials and research below.
Cerebral Palsy impacts approximately 40% of the HIE community. We’ve compiled some resources to look into clinical trials and research studies.
Find a Study on Cerebral Palsy
Select one of the following links to get ClinicalTrials.gov search results for studies on cerebral palsy:
Cerebral Palsy Research Network – United States

CP Research Network focuses on optimizing the lifelong health and wellness of people with cerebral palsy and their families through high quality research, education and community programming.
CPRN has both the patient/family inputted MyCP platform and a network of 30 sites in the United States coordinating research.
Cerebral Palsy Alliance – Australia & New Zealand

Cerebral Palsy Alliance is a ground-breaking, global centre of expertise for cerebral palsy services and support, research, technology and innovation, and advocacy. Their alliance of great minds work together to deliver a world of opportunity for people with cerebral palsy and similar disabilities, and their families.
Select one of the following links to get ClinicalTrials.gov search results for studies on autism spectrum disorder (ASD):
Hope for HIE doesn’t guarantee the accuracy or validity of the information contained in the links below, but hope they will be a helpful starting point to learn more about decision-making and global trials.
Clinical Trial Resources:
Clinical Trial Databases:
Questions? Suggestions? Have an open or upcoming trial or study you’d like us to include?
Connect with families, read inspiring stories, and get helpful resources delivered right to your inbox.